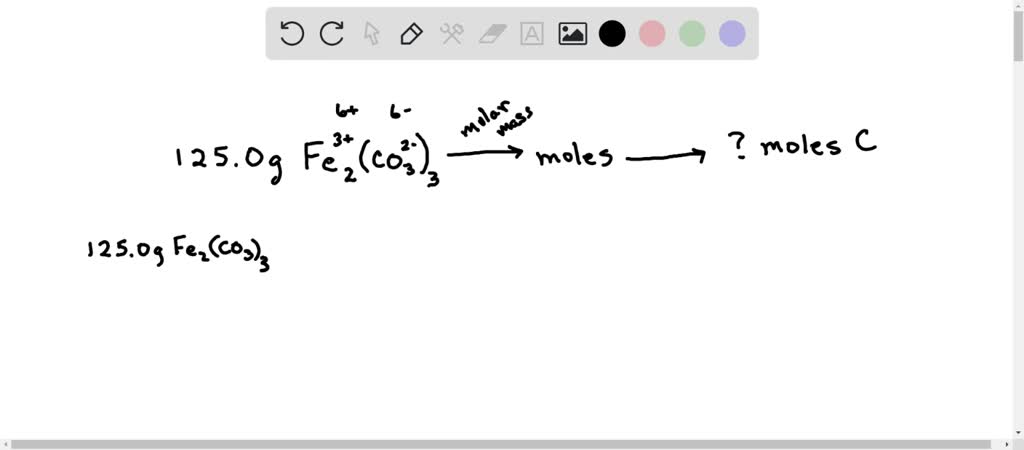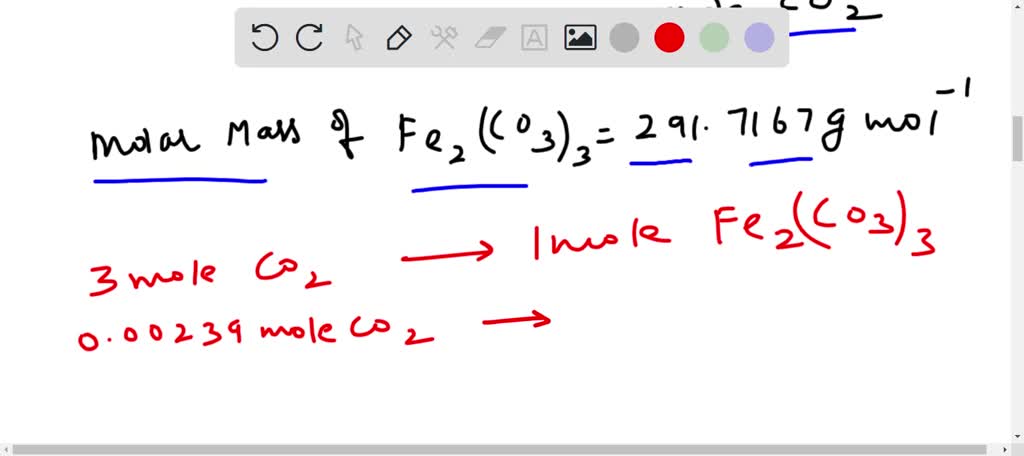
SOLVED: How many grams of iron(III) carbonate decompose to give 53.6 mL of carbon dioxide gas at STP? Fe2(CO3)3??Fe2O3(s)+3CO2(g)
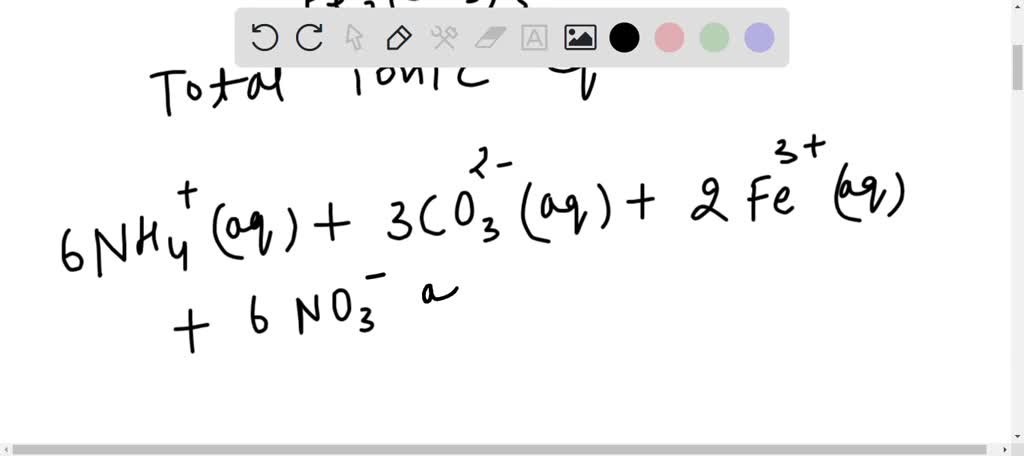
SOLVED: Ammonium carbonate and iron(iii) nitrate are combined, solid iron( iii) carbonate and a solution of ammonium nitrate are formed. the net ionic equation for this reaction is: