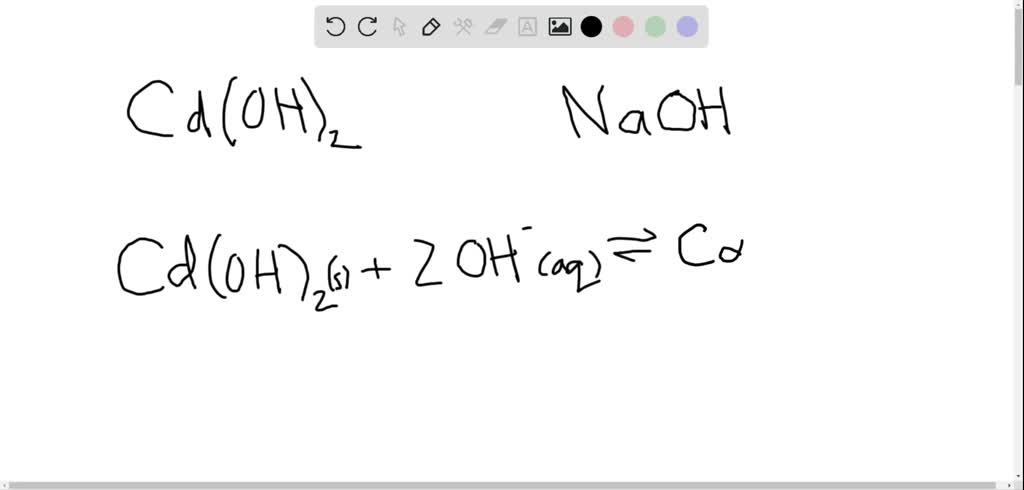
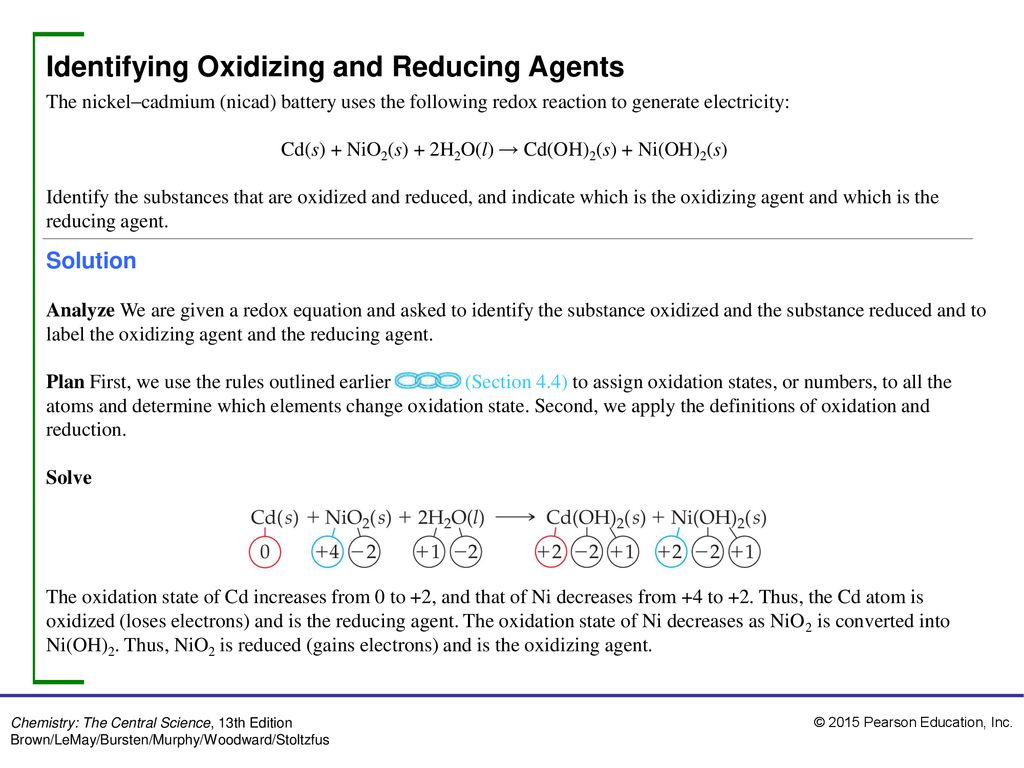
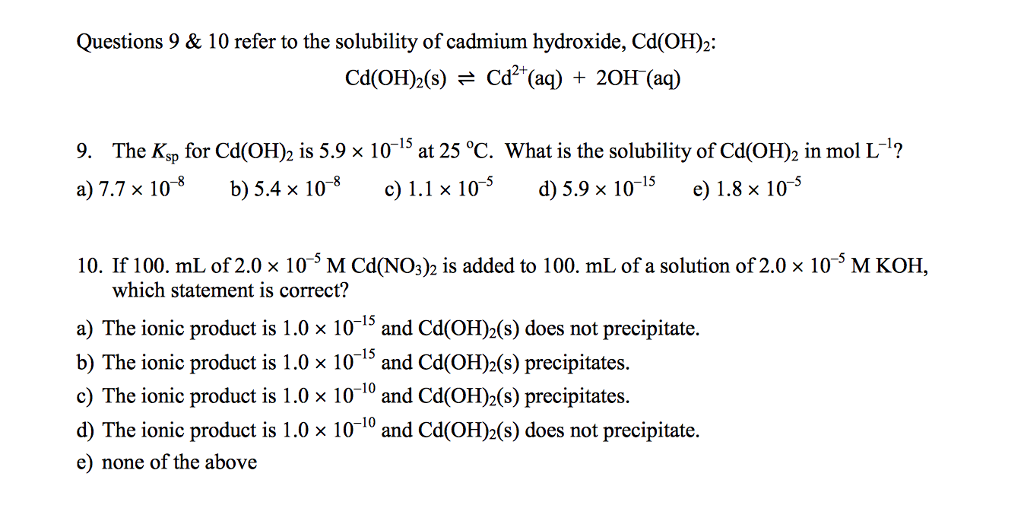
SOLVED: 3 Cadmium hydroxide (Cd(OH)z) is a sparingly soluble salt with a Ksp value of 7.2X 10-15. a) What is the solubility of Cd(OH)z in pure water? b) Whatis the solubility of

Solubility of `Cd(OH)_(2)` in pure water is `1.84xx10^(-5)\"mole\"//L` Calculate its solubility in - YouTube
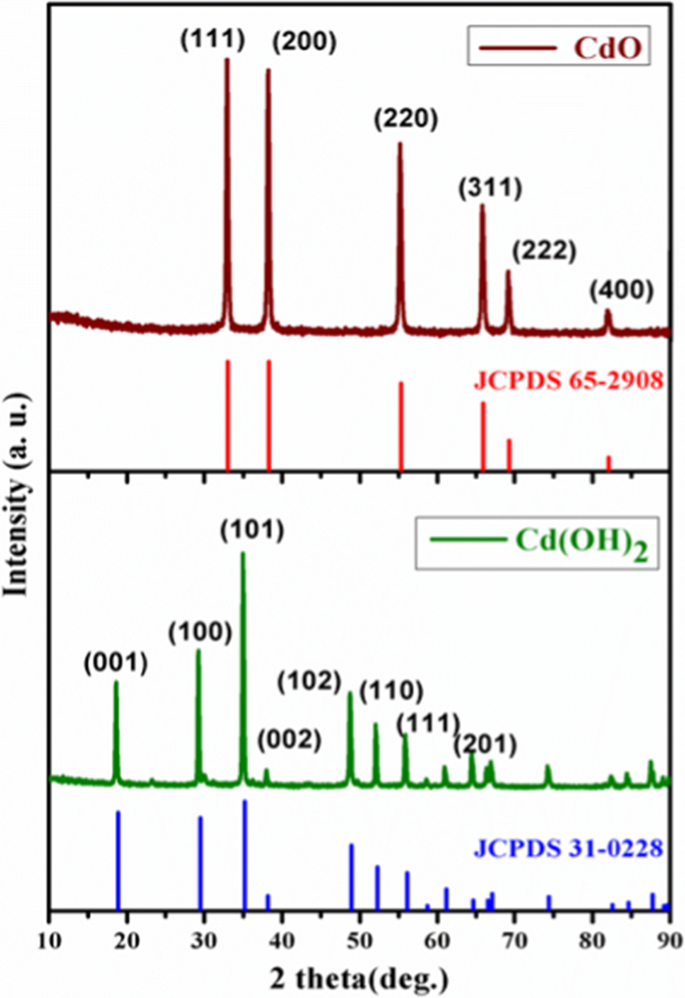
Cd(OH)2 and CdO: structural, optical, electron density distribution analysis with antibacterial assay | SpringerLink
The molar solubility of Cd(OH)2 is 1.84 × 10^–5 M in water. The expected solubility of Cd(OH)2 in a buffer solution of pH = 12 is : - Sarthaks eConnect | Largest Online Education Community
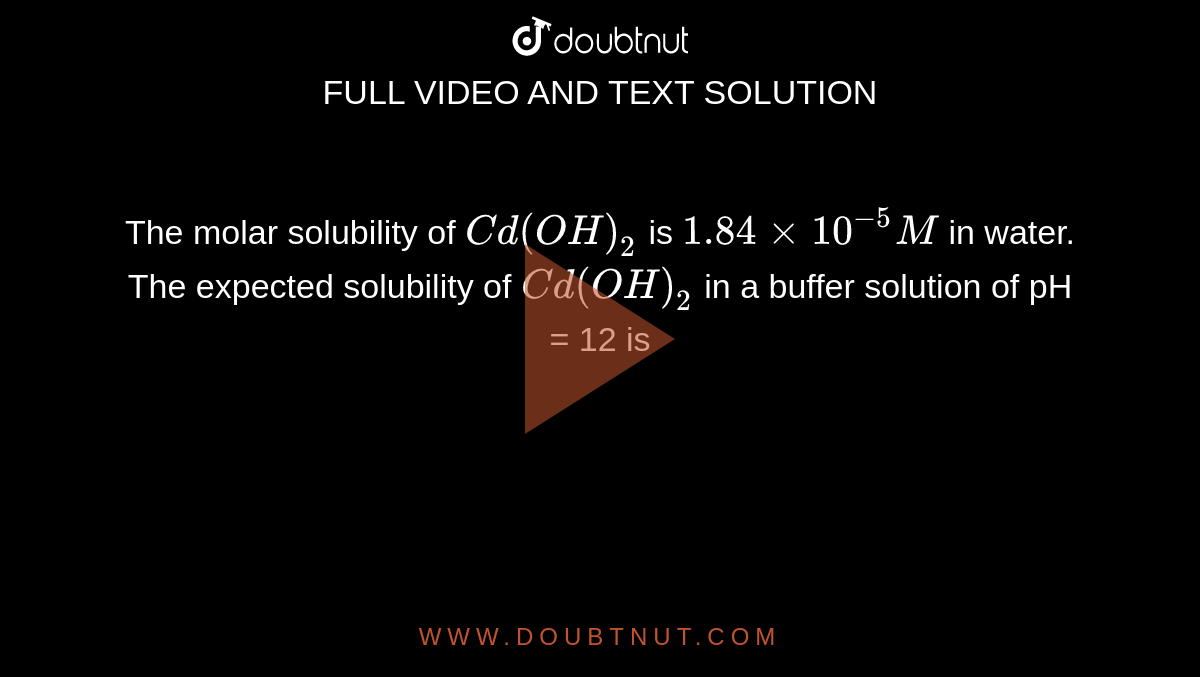
The molar solubility of Cd(OH)(2) is 1.84xx10^(-5)M in water. The expected solubility of Cd(OH)(2) in a buffer solution of pH = 12 is

Buy high pure Cadmium hydroxide Cd(OH)2 Cd(OH) 99.9% chemical compound material 99.9% schpm Industrial Grade from Sichuan HPM - ECHEMI
![What is the solubility of Cd(OH)2 in a buffer solution having pH=8 [Ksp of ( Cd(OH)2 is 2.5×10^-14]? - Brainly.in What is the solubility of Cd(OH)2 in a buffer solution having pH=8 [Ksp of ( Cd(OH)2 is 2.5×10^-14]? - Brainly.in](https://hi-static.z-dn.net/files/d7f/53875d97a1774f791777a981053518e7.jpg)
What is the solubility of Cd(OH)2 in a buffer solution having pH=8 [Ksp of ( Cd(OH)2 is 2.5×10^-14]? - Brainly.in
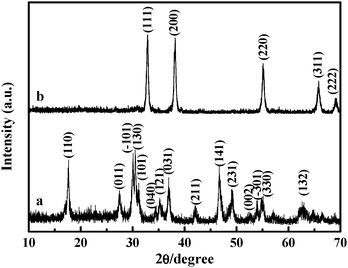
One-step fabrication of Cd(OH)2 nanorings via a solution phase synthesis - Chemical Communications (RSC Publishing) DOI:10.1039/C0CC00665C